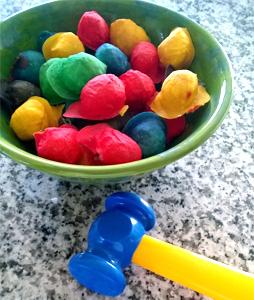
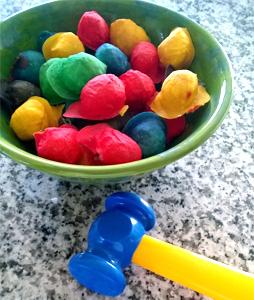
Supplies:
- Cotton balls
- 1 cup flour
- 1 cup water
- Large mixing bowl
- Food coloring
- Baking sheet
- Small bowls or cups
- Either cooking spray or tin foil
- An adult to help with the oven
Directions:
- Mix 1 cup of flour with 1 cup of water in the large bowl.
- Get your baking sheet ready by spraying it with baking spray or covering it with tin foil.
- Divide the flour and water mixture into 4 to 6 small bowls or cups, depending on how many colors you want.
- Add 5 to 8 drops of food coloring to each cup and mix well. Remember that you can make different colors by mixing the food coloring; red and yellow make orange and blue and red make purple.
- Dip each cotton ball into a cup. Be sure to cover the whole cotton ball with the mixture – make it nice and thick.
- Set the coated cotton balls on your baking sheet.
- Let an adult help you with this part. Bake your cotton balls in a 300 degree oven for about 45 minutes.
- After that, take the cotton balls out and let them cool completely – at least an hour. They should have a nice, hard, crunchy shell.
- Find a small hammer, a toy hammer, or even a rock. Take your cotton balls outside and - SMASH THEM WITH THE HAMMER! That’s right – smash away.
Extra fun:
Use more cotton balls to make a baked sculpture. Use the same dipping method, but keep the sculpture in the over a little longer – about 55 minutes. You can make and smash a crunchy monster!
To watch this project visit: https://www.youtube.com/watch?v=Mvw4q6cr6xY&list=PLMEg2Dd0dSFctLfDQxsL5…

Colorful Experiment #1
Supplies:
- Milk
- Plate
- Liquid Food coloring
- Dish Soap
- Q-tips
Directions:
- Pour the milk into a plate until you cover the bottom surface.
- Add drops of food coloring in middle of the milk in the plate.
- Coat the Q-tip in the dish soap and dip it in the milk. Watch what happens!
The science behind this reaction has to do with the way the soap molecules and the fat from the milk are interacting. Fat is hydrophobic, a type of molecule that repels water. By adding the soap, we are breaking up the hydrophobic fat particles and holding it inside the soap.
Colorful Experiment #2
Supplies:
- Hard coated candy
- Plate
- Warm water
Directions:
- Put the candy pieces in the plate. You can place them around the edge, or any other design you can think of!
- Add some warm water to the plate, making sure that there is enough to cover the bottom of the plate. Watch what happens!
The science behind this interaction has to do with the warm water dissolving the color coating on the candies.
Each of the candies has a slight difference in the sugar content, which means they have different densities; they all take up a different amount of space. The reaction we are seeing here is called stratification, where water splits due to differences in the density of the materials.Try cold water and even different kinds of candy. What happens?
Watch these projects at: https://www.youtube.com/watch?v=9geJ7KdXqK0&list=PLMEg2Dd0dSFctLfDQxsL5…

Black Lives Matter and so we celebrate Black Voices in stories for children. These picture books are available at the Pikes Peak Library District. Click on the pdf link below to see the booklist.


